Скачать с ютуб The challenges of developing treatments for rare diseases в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок The challenges of developing treatments for rare diseases в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно The challenges of developing treatments for rare diseases или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон The challenges of developing treatments for rare diseases в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса ClipSaver.ru
The challenges of developing treatments for rare diseases
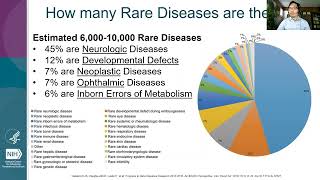
To mark International Rare Disease Day, the BIA is shining a light on the challenges involved in developing drugs for rare diseases. There are around 7,000 known rare diseases yet only 5% of these have a single licensed treatment. Though the development journey is often fraught with challenges, the life-changing benefits these treatments can provide to patients and their families make the development of treatments for rare diseases hugely worthwhile. Industry remains committed to developing medicines across a breadth of disease area. To learn more about the BIA's Rare Disease Industry Group (RDIG), visit www.bioindustry.org/rdig *** Sources used: NORD, Rare Disease Day: Frequently Asked Questions, 2019. Available online via: https://rarediseases.org/wp-content/u... Cancer Research UK, How long a new drug takes to go through clinical trials. Available online via: https://www.cancerresearchuk.org/abou... Seyhan, A., Lost in translation: the valley of death across preclinical and clinical divide, 2019. Available online via: https://transmedcomms.biomedcentral.c... Thomas, K., The price of health: the cost of developing new medicines, 2016. Available online via: https://www.theguardian.com/healthcar... DiMasi, J., Grabowski, H. and Hansen, R., Innovation in the pharmaceutical industry: New estimates of R&D costs, 2016. Available online via: https://pubmed.ncbi.nlm.nih.gov/26928...