Скачать с ютуб Emission & Absorption Spectrum | Structure of Atom | Class 11th & 12th | Science в хорошем качестве
emission and absorption spectra
emission and absorption spectrum class 11
emission an absorption spectra chemistry
atomic spectra
spectroscopy
line apectra
emission and absorption spectra animation
emission and absorption spectra hindi
emission and absorption spectra class 12
emission and absorption spectra studycity
emission and absorption spectrum study city
emission and absorption spectrum vedantu
emission and absorption spectra physicswallah
Скачать бесплатно и смотреть ютуб-видео без блокировок Emission & Absorption Spectrum | Structure of Atom | Class 11th & 12th | Science в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Emission & Absorption Spectrum | Structure of Atom | Class 11th & 12th | Science или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Emission & Absorption Spectrum | Structure of Atom | Class 11th & 12th | Science в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Emission & Absorption Spectrum | Structure of Atom | Class 11th & 12th | Science
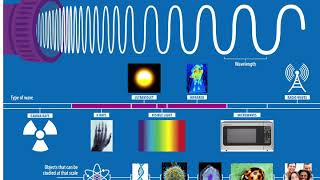
Emission & Absorption are the terms of the chapter "Structure of Atom", in which we learn about Absorbing and Emission Mechanism in the interaction of light with matter. Follow me: Instagram- @abhijeetchobey instagram.com/abhijeetchobey Twitter- @abhijeetchobey twitter.com/abhijeetchobey Light, in its mesmerizing journey through the universe, interacts with matter in a captivating way. This interaction unveils hidden truths about the composition of stars, the workings of neon signs, and even the fundamental structure of atoms. Two key concepts lie at the heart of this dance between light and matter: emission spectra and absorption spectra, which is also called emission spectrum & absorption spectrum. Understanding these phenomena equips us with powerful tools for analyzing our surroundings. An emission spectrum is a unique fingerprint of an element or molecule. It arises when an atom is energized, often through heat or electricity. This energy boosts electrons to higher energy levels within the atom. However, these excited states are unstable, and the electrons quickly jump back down to lower energy levels. As they do, they release energy in the form of light, with specific wavelengths characteristic of the element's electronic structure. The emission spectrum appears as a series of bright lines at these particular wavelengths, like colorful bars on a graph. Conversely, an absorption spectrum reveals the story from the perspective of light. When white light, a combination of all visible wavelengths, passes through a gas or a cool liquid, some wavelengths are absorbed by the atoms or molecules present. This absorption corresponds to the energy required to excite the electrons in those specific elements. The resulting spectrum shows a continuous range of light with dark gaps at the wavelengths that were absorbed. These dark gaps act as a mirror image of the emission lines, revealing the elements that are present in the material. #atomicspectrum #structureofatom #class11th ALL PARTS OF THIS CHAPTER: Part 1 (Subatomic Particles) • Subatomic Particles | Structure of At... Part 2 (Discovery of Electrons) • Discovery of Electron | Structure of ... Part 3 (Charge to mass Ratio of Electron) • Charge to Mass Ratio of Electron | Th... Part 4 (Discovery of Neutrons) • Discovery of Neutrons | Structure of ... Part 5 (Discovery of Protons) • Discovery of Protons | Structure of A... Part 6 (Thomson Model of Atom) • Thomson Model of Atom | Plum Pudding ... Part 7 (Rutherford's Model of Atom) • Rutherford Model of Atom | Scattering... Part 8 (Rutherford's Model Pos. & Lim.) • Rutherford Model of Atom | Postulates... Part 9 (Atomic Number & Mass Number) • Atomic Number & Mass Number | Structu... Part 10 (Isobars & Isotopes) • Isobars & Isotopes | Structure of Ato... Part 11 (Developments Leading to Bohr Model Atom) • Developments leading to the Bohr's Mo... Part 12 (Wave Nature of Electromagnetic Radiation) • Wave Nature of Electromagnetic Radiat... Part 13 (Planck's Quantum Theory) • Particle Nature of E.M Radiation | Pl... Part 14 (Photoelectric Effect Part-1) • Photoelectric Effect | Structure of A... Part 15 (Photoelectric Effect Part-2) • Photoelectric Effect | Structure of A... Part 16 (Dual Behaviour of E.M. Radiation) • Dual Behaviour of Electromagnetic Rad... Part 17 (Atomic Spectrum) • Atomic Spectrum | Structure of Atom |... Part 18 (Emission & Absorption Spectrum) • Emission & Absorption Spectrum | Stru... Part 19 (Line Spectrum of Hydrogen Atom) • Line Spectrum of Hydrogen | Structure... Part 20 (Bohr's Model of Hydrogen Atom) • Bohr's Model of Hydrogen Atom | Struc... Part 21 (Dual Behavior of Matter) • Dual Behaviour of Matter | Structure ... Part 22 (Heisenberg's Uncertainty Principle) • Heisenberg's Uncertainty Principle | ... Part 23 (Pauli's Exclusion Principle) • Pauli's Exclusion Principle | Structu... So, see you in the next video !