Скачать с ютуб GC-MS For Beginners (Gas Chromatography Mass Spectrometry) в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок GC-MS For Beginners (Gas Chromatography Mass Spectrometry) в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно GC-MS For Beginners (Gas Chromatography Mass Spectrometry) или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон GC-MS For Beginners (Gas Chromatography Mass Spectrometry) в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
GC-MS For Beginners (Gas Chromatography Mass Spectrometry)
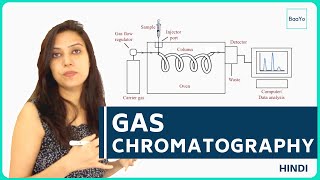
Gas chromatography mass spectrometry is the combination of two techniques we have already covered on the channel, namely and perhaps a bit obviously gas chromatography and mass spectrometry (DUH). The gas chromatography first separates the different components of a mixture and these components are then further separated based on their mass-to-charge ratio. The subsequent results from a gas chromatography mass spectrometer is therefore 3-dimensional, providing mass spectra that can be used for identity confirmation, to identify unknown analytes and to determine structural and chemical properties of molecules, as well as the chromatogram that can be used for qualitative and quantitative analysis. Before we dive into how to read these chromatograms, let us examine how the process is carried out. First we have to zoom in on the gas chromatograph part. In a nutshell it is very simple and works based on the same principles as all other column chromatographies. Its main components are the mobile phase, a molecular sieve, the column and within it the stationary phase in addition to a detector. GC is carried out in the following manner: 1. The sample is first introduced into the gas chromatograph (GC) right before the column, often using a syringe 2. The molecules are separated based on how they interact with the stationary phase. Like separates like, therefore non-polar columns are good for separating non-polar analytes and polar columns are good for separating polar analytes. 3. Then once the different components of the sample exits the column, they are detected and the results are displayed in a gas chromatogram, BUT in addition they also enter the mass spectrometry part of the device for further analysis. Then it is time for the mass spectrometer to do its magic. Although all mass spectrometers ionize and then separate the inserted sample based on its mass-to-charge ratio the way in which they do so actually varies quite a lot. In the case of GC-MS, one common mass spectrometry method is quadrupole mass spectrometry. In a nutshell, the sample is first ionized using electron ionization which basically is just bombarding the sample with electrons until it ionizes. Then the sample enters the mass analyzer part of the device. Here 2 electrical fields caused by the 4 parallel rods start affecting the ionized sample. These magnetic fields cause the ions to oscillate depending on their mass-to-charge ratio. By varying the strength of these 2 magnetic fields, ions of different sizes can be selected by the mass analyzer. As stated, I have covered both how gas chromatography and quadrupole mass spectrometry work in more detail in previous videos so I will link both of them in the description of this one. Please check them out if you wish to understand either of these techniques better. Also, remember to like the video while you are there!! Now as promised, let us examine how to interpret the results from a GC-MS device. Much like with the rest of this device it is merely a question of adding the two results displaying techniques on top of another. Starting with the gas chromatogram, it displays peaks of the generated current from the detector against retention time. In other, words how much sample is exiting the column, how long does it take the sample component to start exiting the column from the time the it was inserted into it and for how long does it exit the column from the time it starts exiting it. The area under the peak gives us information about the concentration of the sample. More area, more concentration and less area, less concentration. Then the mass spectrometry provides another dimension in which we can further analyze these different peaks. By selecting any one of the peaks in the gas chromatogram we get a mass spectra graph of that specific peak with detailed information of the mass-to-charge ratio of the constituent components that make up that peak. This way, even when we do not have previous information about a specific peak in the gas chromatogram we can utilize the mass spectra graph from the mass spectrometry part of the device to determine the specific substance in question.