Скачать с ютуб NCERT Science Class 7 Chapter 5: Acid, Base, Salts | CBSE | English | NSO | Chemistry | Olympiad в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок NCERT Science Class 7 Chapter 5: Acid, Base, Salts | CBSE | English | NSO | Chemistry | Olympiad в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно NCERT Science Class 7 Chapter 5: Acid, Base, Salts | CBSE | English | NSO | Chemistry | Olympiad или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон NCERT Science Class 7 Chapter 5: Acid, Base, Salts | CBSE | English | NSO | Chemistry | Olympiad в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса ClipSaver.ru
NCERT Science Class 7 Chapter 5: Acid, Base, Salts | CBSE | English | NSO | Chemistry | Olympiad
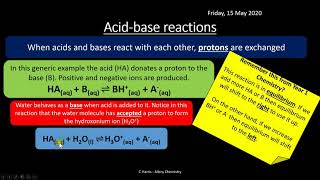
SOF NSO - https://www.doorsteptutor.com/Exams/NSO/ Unified Council NSTSE Preparation - https://www.doorsteptutor.com/Exams/N... CBSE: https://www.doorsteptutor.com/Exams/C... NTSE: https://www.doorsteptutor.com/Exams/N... Practice and Prepare @ https://www.doorsteptutor.com/ Lectures on Various Subjects like Science, Social Studies and Humanities subscribe / testprep Examrace Lectures in Hindi subscribe / examracehindi For kids videos nursery to Class 5 subscribe / funprof For Science (Physics, Chemistry, Biology and Mathematics) Class 11-12 and important topics subscribe / doorsteptutor Also visit NCERT Solutions, NIOS @ https://www.flexiprep.com/ IGCSE, A & AS Level @ https://www.examtestprep.com/ Acid: Organic: Naturally in plants and animals Inorganic or mineral – HCl, HNO3, H2SO4 Strong acid – highly corrosive HCl, HNO3, H2SO4 – causes burns ; stored in glass bottles Weak acid – not that corrosive - citric acid (𝐶_6 𝐻_8 𝑂_7 ), Acetic acid (𝐶𝐻_3 𝐶𝑂𝑂𝐻), oxalic acid (𝐶_2 𝐻_2 𝑂_4 ) Concentrated – small amount of water Dilute – more amount of water - 3% Hydrochloric acid is an example of dilute acid - Most acids used in the laboratory are diluted with water because their concentrated form can be too dangerous to handle Bases Examples of strong bases are sodium hydroxide (𝑁𝑎𝑂𝐻) and potassium hydroxide (𝐾𝑂𝐻). bases are corrosive in nature and should be handled with care Weak bases are not as corrosive as strong bases. Magnesium hydroxide [𝑀𝑔(𝑂𝐻)_2 ], ammonium hydroxide [𝑁𝐻_4 𝑂𝐻] and copper hydroxide [𝐶𝑢(𝑂𝐻)_2] are a examples weak bases Some bases, but not all, can dissolve in water. Bases that dissolve in water are called alkalis. Sodium hydroxide (𝑁𝑎𝑂𝐻) and potassium hydroxide (𝐾𝑂𝐻), are examples of alkalis. Example – soap, detergent, drain cleaner, antacids, fertilizers like ammonium hydroxide; KOH in batteries and fertilizers Neutralization When phenolphthalein is added to the acid solution in the flask, it remains colourless. As the base is added to the acid, after a certain point, the solution turns pink. Change in colour of the solution to pink indicates that the entire volume of the acid has undergone neutralization by the base and the salt has been formed. Indigestion: Antacids such as Milk of Magnesia (magnesium hydroxide) and baking soda (sodium hydrogen carbonate) are used to neutralize the excess acid, relieving the symptoms of indigestion. Ant Bite: The acid is neutralized by rubbing moist baking soda (sodium bicarbonate), calamine solution (containing zinc carbonate) or even toothpaste on the area. Soil: Acidic soil can be neutralized using slaked lime or quicklime, which is a base. If the soil is too basic, organic matter is added to it. Waste Water: Slaked lime or calcium hydroxide is often used to neutralize the acidic substances in the effluent. Neutral Substance: Salts, most cosmetics, lotions, eye drops have pH of 7 - Safe to ingest and leave on skin. Chapters: 0:00 NCERT Science Chapter 5 Class 7- Acid, Base, Salts 0:18 Acids 7:27 Sources of Acids 8:14 Plants and Animal Acids 8:30 Organic and Inorganic Acids 8:56 Testing Acids 10:04 Bases 10:40 Strong and Weak Base 12:50 Alkali 15:47 Indicators 28:15 Neutralization 32:24 Salts 34:47 Acidic, Basic and Neutral Salts 35:24 Uses of Salts #ncertsciencechapterfiveclasssevenacidbasesalts #acids #sourcesofacids #plantsandanimalacids #oragnicandinorganicacids #testingacids #bases #strongandweakbase #alkali #indicators #neutralization #salts #acidicbasicneutralsalts #usesofsalts #examrace NCERT Science Chapter 5 Class 7, Sources of Acids, Neutralization, Acidic, Basic and Neutral Salts, ncert examrace, ncert history, ncert class 6, upsc ncert, ias ncert, ncert by manishika jain, Alkali #NSO #NSTSE #Olympiad #NCERT #VVM #examrace #upsc #ugcnet