Скачать с ютуб Chemistry Experiment: Does Alka-Seltzer Tell the Truth? в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Chemistry Experiment: Does Alka-Seltzer Tell the Truth? в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Chemistry Experiment: Does Alka-Seltzer Tell the Truth? или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Chemistry Experiment: Does Alka-Seltzer Tell the Truth? в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
Chemistry Experiment: Does Alka-Seltzer Tell the Truth?
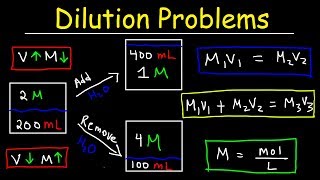
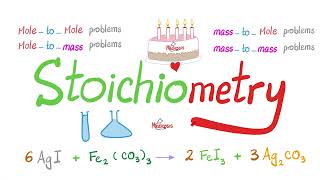
In this video we will experimentally determine the amount of sodium bicarbonate in an effervescent tablet. We will perform the reaction and measure the mass of carbon dioxide that is lost. Then, we'll use stoichiometry to calculate the amount of sodium bicarbonate and compare it to the package. Here's the procedure: Purpose Determine the amount of sodium bicarbonate in an effervescent tablet. Materials Effervescent tablet Vinegar Large cup or jar Electronic balance or kitchen scale (preferably with 0.01 g precision) Small square or wax or parchment paper Procedure Pour 35 mL of vinegar into the cup or jar. Place the cup with vinegar on the balance and record the mass in table 1. Place the piece of wax paper on the balance and zero the balance. Put the tablet on the wax paper and record the mass in table 1. Add the mass from step 1 and step 2 and record in table 1 as the mass before reaction. (show your work in the space below the data table) Carefully drop the tablet into the vinegar and allow the reaction to go to completion (once the bubbling stops completely, the reaction is over). Place the cup on the balance and record the mass in table 1 as the mass after reaction. Subtract the mass after the reaction from the mass before the reaction and record in table 1 as the mass of CO2. (show your work in the space below the data table) Thanks for watching! Support The Science Classroom by 'liking' and 'subscribing' #chemistry #chemed #experiment #scienceexperiment #science Contents Intro 0:00 How to solve Stoichiometry Problems 1:10 Experiment 4:17