Скачать с ютуб Synthesis of Drugs: Encorafenib в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Synthesis of Drugs: Encorafenib в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Synthesis of Drugs: Encorafenib или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Synthesis of Drugs: Encorafenib в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса savevideohd.ru
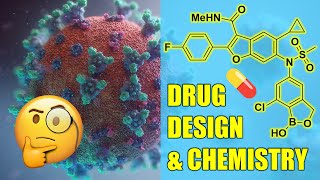
Synthesis of Drugs: Encorafenib
Encorafenib is an oncology drug for the treatment of late-stage (metastatic) melanoma that was approved by the FDA in 2018. It is currently marketed in the US by Pfizer. A scalable synthesis of Encorafenib was disclosed by researchers at IRM LLC and Novartis AG. It is a chiral compound incorporating a key trisubstituted-pyrazole scaffold which is a structural feature of several potential antiproliferative agents. The molecule also incorporates a pyrimidine heterocycle and a fluorinated phenyl ring. Chemical steps: Synthesis of building block #1: 1. Condensation with benzyl chloroformate. 2. Addition of methyl chloroformate delivers the bis-carbamate. 3. Hydrogenation with palladium on carbon provides the chiral BB1. Synthesis of building block #3: 1. Formylation reaction delivers the benzaldehyde. 2. Oxidation to the carboxylic acid. 3. Curtius rearrangement. • Curtius Rearrangement . 4. Pd-catalyzed borylation under Miyaura conditions. Synthesis of building block #2: 1. Benzyl protection of the hydrazine. 2. Alkylation reaction and acid-promoted cyclization. 3. Methyl ketone formation. 4. Sandmeyer reaction. • Sandmeyer Reaction . 5. Condensation reaction and cyclization with guanidine. 6. Conversion of the amine to the pyrimidone. 7. Chlorination with phosphorus oxychloride. Synthesis of Encorafenib: 1. Nucleophilic aromatic substitution, SNAr. 2. Suzuki cross-coupling reaction. • Suzuki Coupling . 3. Removal of the Boc protecting group. Double mesylation of the amine. And removal of the extra mesyl moiety. References: Andrew C. Flick, Carolyn A. Leverett, Hong X. Ding, Emma McInturff, Sarah J. Fink, Christopher J. Helal, Jacob C. DeForest, Peter D. Morse, Subham Mahapatra, and Christopher J. O’Donnell, J. Med. Chem. 2020, 63, 19, 10652–10704. https://doi.org/10.1021/acs.jmedchem.... H. Mei, J. Han, S. Fustero, M. Medio-Simon, D. M. Sedgwick, C. Santi, R. Ruzziconi, V. A. Soloshonok, Chem. Eur. J. 2019, 25, 11797. https://doi.org/10.1002/chem.201901840 H. Mei, J. Han, S. White, D. J. Graham, K. Izawa, T. Sato, S. Fustero, N. A. Meanwell, V. A. Soloshonok, Chem. Eur. J. 2020, 26, 11349. https://doi.org/10.1002/chem.202000617