Challenges of PTH Testing in Patients with CKD скачать в хорошем качестве
Повторяем попытку...
Скачать видео с ютуб по ссылке или смотреть без блокировок на сайте: Challenges of PTH Testing in Patients with CKD в качестве 4k
У нас вы можете посмотреть бесплатно Challenges of PTH Testing in Patients with CKD или скачать в максимальном доступном качестве, видео которое было загружено на ютуб. Для загрузки выберите вариант из формы ниже:
-
Информация по загрузке:
Скачать mp3 с ютуба отдельным файлом. Бесплатный рингтон Challenges of PTH Testing in Patients with CKD в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием видео, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса ClipSaver.ru
Challenges of PTH Testing in Patients with CKD
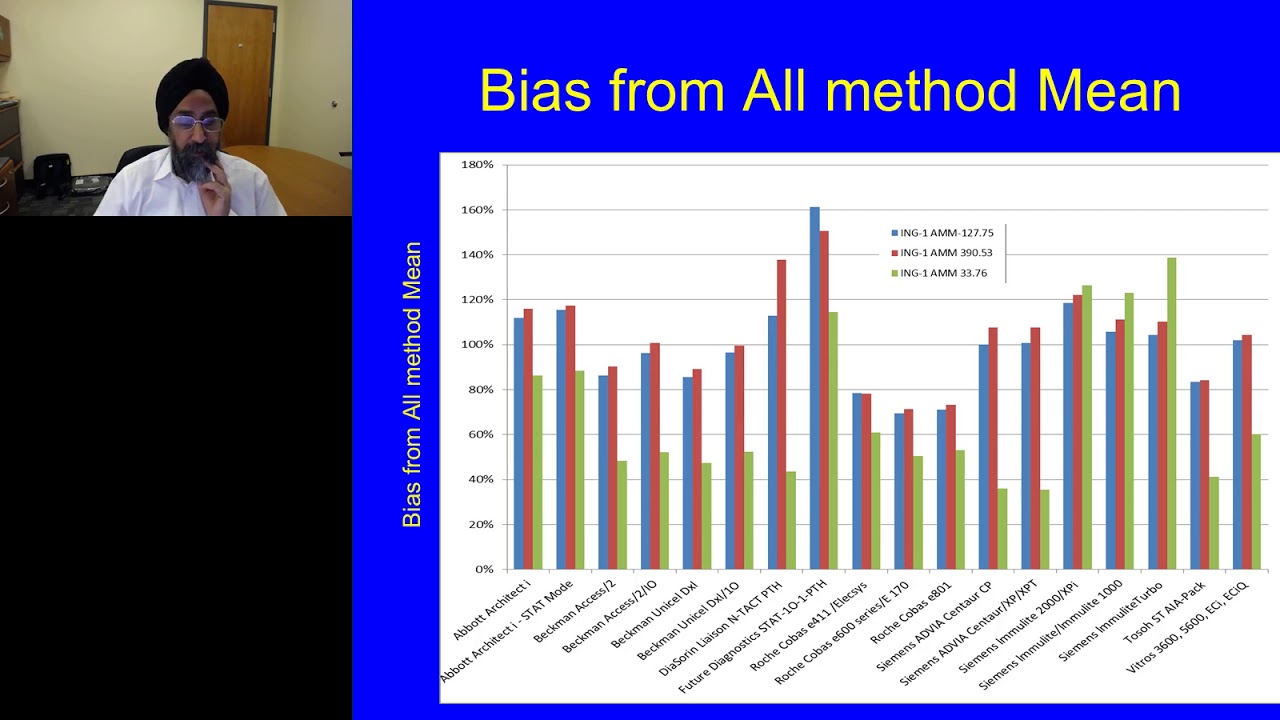
Presented At: LabRoots - Clinical Diagnostics Virtual Event 2018 Presented By: Ravinder Singh, PhD - Director, Endocrine Laboratory, Mayo Clinic Speaker Biography: Ravinder J. Singh, Ph.D., is the director of the Mayo Clinic Endocrine Laboratory. He has a focused area of investigation that has broad applicability to the field. Dr. Singh studies the application of liquid chromatography-tandem mass spectrometry (LC-MS/MS) to clinical laboratory analysis. Many of the methods that Dr. Singh developed are now considered reference methods. They have subsequently been utilized for method standardization efforts as well as to establish clinical disease correlates, which he has published with his collaborators. Dr. Singh's work has directly contributed to improving methods for the clinical diagnosis of Cushing's disease, pheochromocytoma and congenital adrenal hyperplasia. He continues to work to discover innovative ways to better understand the uses of LC-MS/MS in providing patients with faster and more accurate diagnoses. Webinar: Challenges of PTH Testing in Patients with CKD Webinar Abstract: Second and third generations of PTH immunoassays currently available on the market demonstrate significant variability with up to 4.2 fold difference in measurements depending on the method used. Such significant variability is due to differences in antibodies specificity, lack of proper calibration or potential interferences. Pre-analytical conditions, such as sampling and storage, also play significant role in stability of PTH. The measurement inconsistencies between methods, as well as pre-analytical challanges complicate establishment of treatment and prevention cutoff values. The main effort of the PTH IFCC working group is to improve calibration of currently available assays using the available reference material. The development of mass spectrometry-based reference measurement procedure is also underway. Ideally, the reference method should allow for differentiation of intact PTH (1-84) and its fragments. Standardization of PTH will provide an opportunity for accurate diagnostic and improved pathophysiologic insight into CKD-MBD, hypo- and hyperparathyrioidism. Earn PACE/CME Credits: 1. Make sure you’re a registered member of LabRoots (https://www.labroots.com/virtual-even...) 2. Watch the webinar on YouTube above or on the LabRoots Website (https://www.labroots.com/virtual-even...) 3. Click Here to get your PACE (Expiration date – November 14, 2020 01:30 PM) – https://www.labroots.com/credit/pace-... LabRoots on Social: Facebook: / labrootsinc Twitter: / labroots LinkedIn: / labroots Instagram: / labrootsinc Pinterest: / labroots SnapChat: labroots_inc









