Скачать с ютуб Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool в хорошем качестве
atomic number and mass number
atomic number
mass number
atomic number chemistry
atomic mass number
Atomic Number
what is atomic mass
mass number chemistry
what is atomic number
what is mass number
atom chemistry
GCSE chemistry revision
atom chemistry fuseschool
what is atomic mass number
atomic mass number revision
fuseschool chemistry playlist
fuseschool global education
properties of matter chemistry
Скачать бесплатно и смотреть ютуб-видео без блокировок Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса ClipSaver.ru
Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool
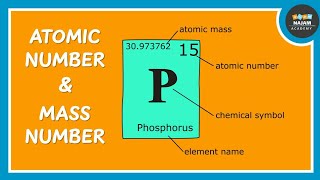
Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool How do we tell elements apart from each other? Find out in this video from the Properties of Matter chapter of FuseSchool GCSE Chemistry. When you look at the periodic table you will see that each element has its own box, and within that box, you will find two numbers. The atomic number, or proton number, and the mass number, but what do these numbers mean? The atomic number has the symbol 'z', this number tells you how many protons are in one atom of an element. The number is always the same for all atoms of a particular element. Atoms of different elements have different atomic numbers, meaning they have different numbers of protons. For example, an atom of Hydrogen has an atomic number of 1 because it has 1 proton, but an atom of Oxygen has and atomic number of 8 because it has 8 protons. The next number we look at is the mass number. The mass number has the symbol A. The mass number tells you how many protons and neutrons are in one atom of an element. We need to remember that Protons and Neutrons each have a relative mass of 1 and that Electrons are so small, that their mass does not need to be considered in the mass number of an atom. So if we know the mass number of an element, and we know the atomic number, we can calculate the number of Neutrons in an atom of a particular element. So the Mass Number = The Atomic Number + the Number of Neutrons. The Atomic Number is just the number of Protons and Atoms, therefore, the Mass Number = protons + neutrons. So if we take oxygen: oxygen has a mass number of 16 and 8 Protons, how many neutrons does it have? Remember, Mass Number = protons + neutrons We can rearrange this to show that Neutrons = Mass Number - Atomic Number. Neutrons = 16 - 8 = 8 Oxygen therefore has 8 neutrons. Let take another example. Lithium has a mass number of 7 and an atomic mass of 3, how many neutrons does it have? Mass Number = protons + neutrons; we can rearrange this to show that Neutrons = Mass Number - Atomic Number. Neutrons = 7 - 3, = 4 Lithium therefore has 4 neutrons. So the atomic number is the number of protons in an atom and the mass number is the number of protons and neutrons in an atom. SUPPORT US ON PATREON / fuseschool SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT. VISIT us at www.fuseschool.org, where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you. These videos can be used in a flipped classroom model or as a revision aid. Find all of our Chemistry videos here: • CHEMISTRY Find all of our Biology videos here: • BIOLOGY Find all of our Physics videos here: • PHYSICS Find all of our Maths videos here: • MATHS Instagram: / fuseschool Facebook: / fuseschool Twitter: / fuseschool Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org Follow us: / fuseschool Befriend us: / fuseschool This is an Open Educational Resource. If you would like to use the video, please contact us: [email protected]