Скачать с ютуб Is HF (Hydrogen fluoride) Ionic or Covalent? в хорошем качестве
breslyn
is HF ionic or covalent
is HF ionic or molecular
ionic or covalent HF
is HF an ionic compound
is HF a covalent compound
is HF a molecular compound
how to tell if HF is ionic or covalent
is Hydrogen fluoride ionic or covalent
how to tell if Hydrogen fluoride is ionic or covalent
is Hydrogen fluoride ionic or molecular
is Hydrogen fluoride an ionic compound
is Hydrogen fluoride a covalent compound
is Hydrogen fluoride a molcular compound
Скачать бесплатно и смотреть ютуб-видео без блокировок Is HF (Hydrogen fluoride) Ionic or Covalent? в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Is HF (Hydrogen fluoride) Ionic or Covalent? или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Is HF (Hydrogen fluoride) Ionic or Covalent? в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса ClipSaver.ru
Is HF (Hydrogen fluoride) Ionic or Covalent?
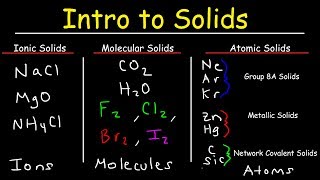
To tell if HF (Hydrogen fluoride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that H is non-metal and F is a non-metal. When we have a non-metal and a non-metal the compound is usually considered covalent. For HF there will is a larger difference in electronegativity between the non-metal and non-metal. This difference results in an electron(s) being shared unequally with the non-metal having a higher electronegativity having the electron more of the time.. --- Helpful Resources Metals, Non-Metals on the P- Table: • Metals, Nonmetals, and Metalloids on ... Ionic, Covalent, & Polar Covalent: • Polar, Non-Polar, and Ionic Compounds... Electronegativity for each element: https://en.wikipedia.org/wiki/Electro... --- Because we have a combination of a non-metal and non-metal HF (Hydrogen fluoride) is considered an covalent compound. For more chemistry help, see http://www.Breslyn.org.