Скачать с ютуб Catecholamine biochemistry - adrenaline/epinephrine & noradrenaline/norepinephrine synthesis, etc. в хорошем качестве
Скачать бесплатно и смотреть ютуб-видео без блокировок Catecholamine biochemistry - adrenaline/epinephrine & noradrenaline/norepinephrine synthesis, etc. в качестве 4к (2к / 1080p)
У нас вы можете посмотреть бесплатно Catecholamine biochemistry - adrenaline/epinephrine & noradrenaline/norepinephrine synthesis, etc. или скачать в максимальном доступном качестве, которое было загружено на ютуб. Для скачивания выберите вариант из формы ниже:
Загрузить музыку / рингтон Catecholamine biochemistry - adrenaline/epinephrine & noradrenaline/norepinephrine synthesis, etc. в формате MP3:
Если кнопки скачивания не
загрузились
НАЖМИТЕ ЗДЕСЬ или обновите страницу
Если возникают проблемы со скачиванием, пожалуйста напишите в поддержку по адресу внизу
страницы.
Спасибо за использование сервиса ClipSaver.ru
Catecholamine biochemistry - adrenaline/epinephrine & noradrenaline/norepinephrine synthesis, etc.
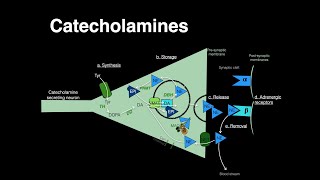
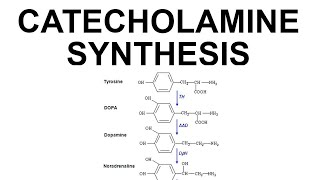
Adrenaline/epinephrine & noradrenaline/norepinephrine can act both as “neurotransmitters” AND “hormones” - the “job title” just refers to whether a chemical messenger is released from a nerve cell or from a gland - it has nothing to do with the actual messenger molecule that’s released. So when noradrenaline is released from nerves in your sympathetic nervous system we call it a neurotransmitter. But when it’s released from your adrenal gland we call it a hormone. Adrenaline is primarily “only” a hormone because it’s almost all released from your adrenal gland, with just a little being made & used by a group of neurons in your brainstem; but noradrenaline is the main neurotransmitter used by the sympathetic nerves in your cardiovascular system. full text: http://bit.ly/adrenalinehormonesetc There are different types of adrenergic receptors (aka adrenoreceptors), different cells express different combos, & they can elicit slightly different responses…So there’s a lot of concerted action that’s coming from that one molecule & there’s no simple answer for what they do. So I’ll leave that level of complexity for hormone & neuro people…. & talk about the biochemical make-up (& making) of these messengers! ⠀ ⠀ Catecholamines come from the amino acid tyrosine (abbreviated Tyr or Y). more on it here: http://bit.ly/tyrosinehormones ⠀ ⠀ Tyrosine’s side chain is the same ring-y thing as that of another amino acid, phenylalanine (Phe, F), with a crucial difference: Tyr has a hydroxyl (-OH) group plopped on. This helps make Tyr & Tyr-based produces soluble so they can travel between neurons & throughout our blood in their chemical messenger duties! Tyrosine can come from your food or from making it from Phe (in fact, about half of the Phe you eat is actually used to make Tyr). (but you can’t make phenylalanine so Phe is “essential” while Tyr is “nonessential” in the dietary sense). ⠀ ⠀ The first step on the path from Tyrosine to adrenaline is adding ANOTHER -OH to the ring to give you L-DOPA. (The L refers to the stereochemistry (which way the atoms stick off from each other - L-DOPA comes from L-tyrosine, which is the “normal” version of tyrosine). This second OH make it even more soluble, & it gives it a dope name - we call benzene-based (6-sided aromatic ring) molecules with 2 -OH groups “catechols” & since it also has an amine (NH₂/NH₃) group, we call L-DOPA & its derivatives CATECHOLAMINES. (so that’s where that name comes from!)⠀ ⠀ Since (-OH) is called a hydroxyl group, we call this “gifting of a hydroxyl group” hydroxylation. Although is it really proper gifting if you steal from molecular oxygen (O₂) to give to the tyr-ed? ⠀ This specific hydroxylation is helped out by an enzyme called Tyrosine Hydroxylase (TH), which is a homotetramer (4 copies of the same protein chain working as a team), which is itself helped out by a cofactor (non-protein helper molecule that the protein enzyme binding to to give it “superpowers”) called TetraHydroBiopterin (THB)⠀ ⠀ L-DOPA is turned into dopamine by removing the carboxyl group - the thing that made an amino acid an acid. But you still have the amino part, & you have the catechol part. So dopamine is still characterized as a catecholamine. This decarboxylation is catalyzed by an enzyme called DOPA DeCarboxylase (DDC). It also decarboxylates other aromatic amino acids, so another name for it is Aromatic Amino acid DeCarboxylase (AADC). This uses a vitamin B6-derived cofactor called PyridoxaL Phosphate (PLP)⠀ ⠀ Now that you have dopamine, what to do? Some cells in the substantial nigra (part of the basal ganglia in your midbrain) stop here and use dopamine as a neurotransmitter as is, where it plays important roles in reward responses. But, otherwise, the next step is turning it into noradrenaline (aka norepinephrine). This is accomplished by adding another OH, but this time to one of the non-ring carbons. And this is catalyzed by Dopamine β-Hydroxylase (DBH). DBH is another tetramer, but a heteromeric one (not all its subunits are the same). And It, too, uses a cofactor, but it uses ascorbate so it’s vitamin-C dependent. ⠀ ⠀ Noradrenaline can be used as-is. Or it can be further processed to form adrenaline by adding a methyl (-CH₃) group onto the end of of it. That’s done by Phenylethanolamine N-MethylTransferase (PNMT), with the extra methyl coming from the cofactor S-AdenosylMethionine (SAM). Only cells with PNMT can do this conversion, and PNMT is not found in most neurons (though it is made from a small group of neurons in the brainstem). Instead, most of it is in the adrenal gland (specifically in the inner part called the medulla) & its activity is regulated by corticosteroids - hormones like cortisol made in the outer part of the adrenal gland (the adrenal cortex)⠀ ⠀ Breaking down catecholamines requires catecholamine-O-methyltranserase (COMT) & monoamine oxidase (MAO). If MAO sounds familiar it’s likely because MAO inhibitors can be used as antidepressants.⠀